TECHNOLOGY OVERVIEW
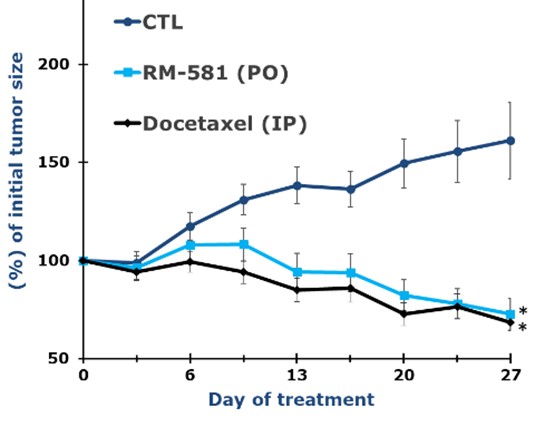
RM-581 is an optimized lead candidate identified from an aminosteroid molecular platform library. This compound has been shown as a potent anticancer agent in vitro and in vivo on a numbers of cancer models, including pancreatic, prostate and triple-negative breast cancers.
Interestingly, RM-581 do not induced drug resistance over time and is very well tolerated by mice at massive dose (>720 mg/kg).
A fluorescent version of RM-581 has been shown to accumulate in the endoplasmic reticulum (ER). This triggers the unfolded protein response (UPR) and related stress apoptosis, through the involvement of GRP78 and STARD5 both found in numerous cancers and contributing to drug resistance. Finally, RM-581 shows a strong synergistic action with antineoplastic agents, suggesting it can also be considered for combined drug therapies.
Remarkably, RM-581 induced tumors regression to a unmeasurable level in mice without any sign of toxicity in resistant cancer xenograft models after only 30 days of treatment.
Currently, RM-581 can be prepared at laboratory multi-gram scale quantities in high purity and in a crystallized form, being ready for translational studies.
COMPETITIVE ADVANTAGES
- Orally active
- New mechanism of action
- Effective as a standalone treatment on a wide range of cancer types
- Synergy with other anticancer agents
- Short chemical synthesis
BUSINESS OPPORTUNITY
- Technology available for in-licensing
- Seeking for industrial partner for co-development
- Eligibility to government financing for industry/academic maturation program
IP PROTECTIOB