TECHNOLOGY OVERVIEW
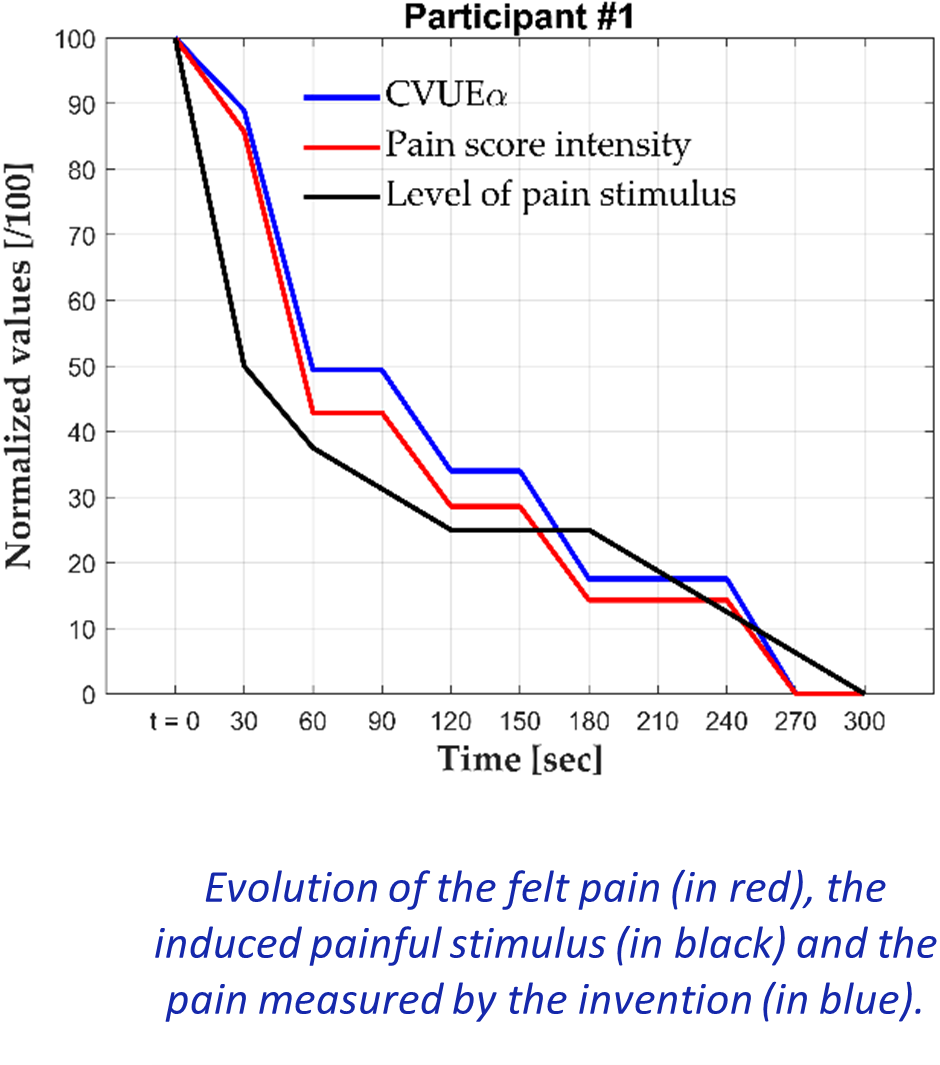
We propose a novel method based on the analysis of electroencephalography (EEG) signals to objectively assess pain level. The invention, the Pain Identification and Quantification (PIQ), is performed into three steps:
1) Transformation of the original real-valued EEG signal into a complex-valued analytical signal
2) extraction of the variations of the instantaneous amplitude of the signal or the amplitude of the upper envelope
3) final calculation based on the coefficient of variation (CV) of the upper envelope
Combined with classical rating scale, PIQ has the potential to become the new reference in pain assessment for consistent results and reproducible clinical trials.
A prototype is fully fonctionnal. The system has been successfully validated in human. It precisely and objectively measures pain levels variation from induced painful stimulus, and its performance with chronic pain is under investigation. This pain assessment method could also be adapted to animal models, and significantly accelerate drug discovery at the pre-clinical and clinical stages, leading to better pain management in a near future.
COMPETITIVE ADVANTAGES
- Objective pain measurement
- Reproducible
- Precise
- Non-invasive
BUSINESS OPPORTUNITY
- Technology available for in-licensing
- Seeking for industrial partner for co-development
- Eligibility to government financing for industry/academic maturation program
MARKET APPLICATIONS
- Drug development (Clinical trials
- Patient monitoring
IP PROTECTION
- Patent Pending (Provisional)